Great discussion here…
so that you can put the new man in a room without mixing sexes
That’s unbelievable!
What will you do when you get the “new system” where a man can temporarily self declare as female, or vice versa? The NHS will get sued by “human rights” lawyers.
Starting to become graphs with vaccine uptake. This graph shows doses per 100 persons. Israel “leads”.
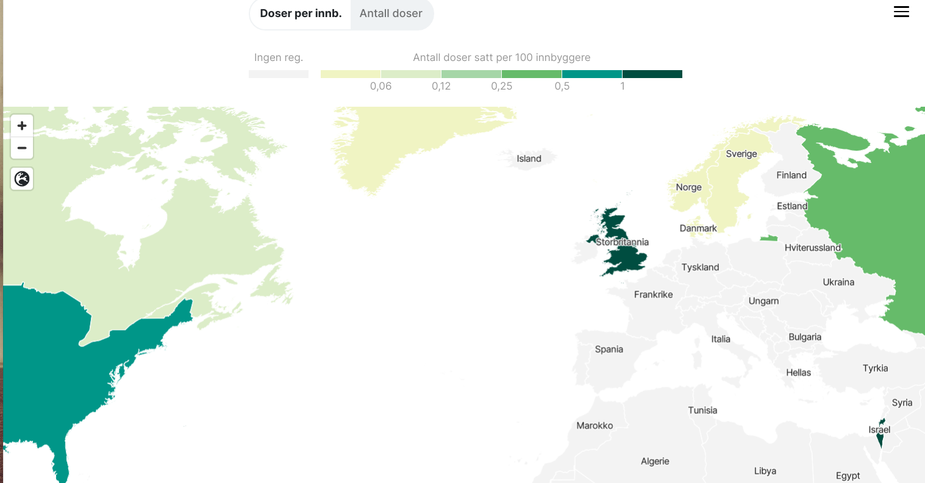
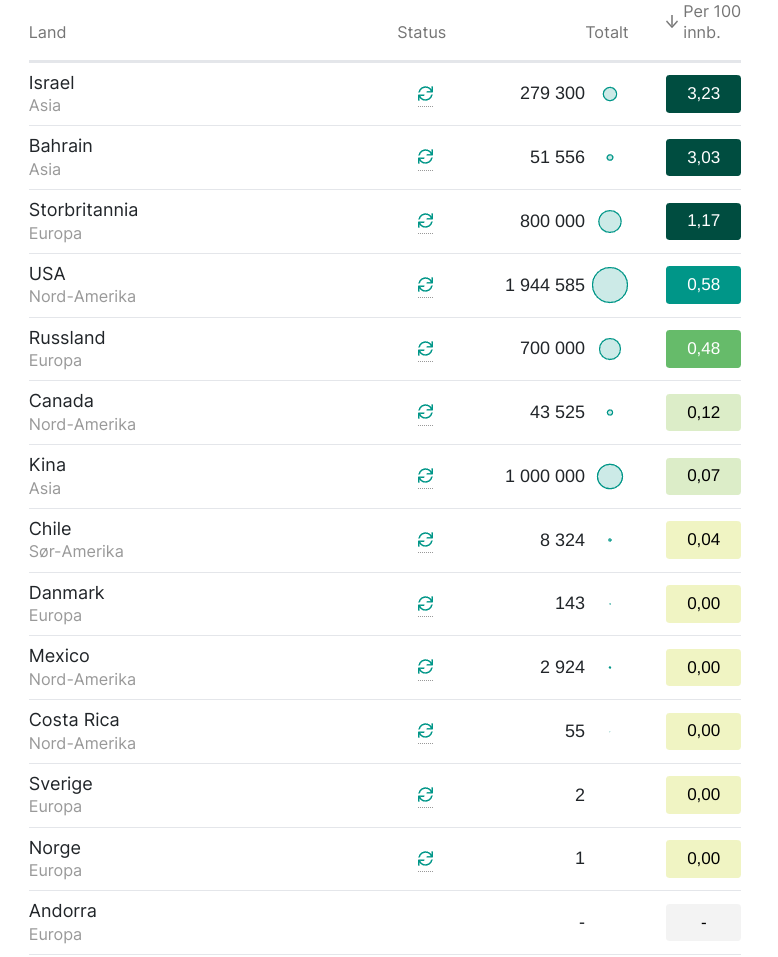
It will be interesting to see how the effect regarding deaths and general spreading will be the next months.
Interesting graph. Good on Israel, the UK and the US to hit the ground running regarding vaccination. In Germany, many people are upset at the government’s inability to secure us a large enough number of doses of the vaccine, invented by a company from our country after all, to vaccinate large parts of the population quickly. I’d say staff wise, we should be able to vaccinate several hundred thousands of people per day, if we put our mind to it, because there are a lot of people in Germany who are trained to do a simple i.m. injection. Vaccinating 27 million against Influenza only took a few weeks this year – despite Covid! – although that only requires a single dose.
Peter wrote:
What will you do when you get the “new system” where a man can temporarily self declare as female, or vice versa? The NHS will get sued by “human rights” lawyers.
The aim would be something pragmatic and respectful to all. I have only looked after a small number of transgender patients, but I think it’s more usual for people to be genuine and longstanding in their identification. It is of course important to keep people safe – there have been assaults in prisons involving transgender people as either victims or aggressors. Some people are more convincingly transitioned than others and it’s in nobody’s interests for someone to be next to an old lady with dementia who shouts ’there’s a MAN in the room. What’s a MAN doing in the room. I don’t want to be in a room with a MAN in it!’ all night, as they fairly often do even if the ‘man’ in question has biologically female since before birth, and socially female ever since. It would be convenient if the hospital was running at 80% capacity and there were some flexibility left.
MedEwok wrote:
Vaccinating 27 million against Influenza only took a few weeks this year – despite Covid! – although that only requires a single dose.
But isn’t the limiting factor the -70° temperature the vaccine has to be kept at, rendering the usual mom and pop doctor useless because he doesn’t have the proper fridge ? As an ex-military officer I know why it doesn’t work. No operation that is centrally organized and that’s not run by the military is bound to fail. Because there are too many non-direct reporting lines involved.
No, I think the limiting factor is still vaccine availability and approval. Also, needing to keep people socially distanced for 15 minutes of observation after vaccination means that you need a lot of space to vaccinate everyone.
I know that there have been problems with care home residents. Either you bring the vaccine to the patient, or the patient to the vaccine, and logistically it is challenging to move care home residents to the vaccine. But for most people it’s simply a matter of availability.
EuroFlyer wrote:
But isn’t the limiting factor the -70° temperature the vaccine has to be kept at, rendering the usual mom and pop doctor useless because he doesn’t have the proper fridge ?
AFAIK, the vaccine can be stored at normal fridge temperatures for up to 6 day, so it is all a question of organisation. GP practices in the UK are already vaccinating patients, and they don’t have the ultra-cold fridges either.
I heard the UK has so far done about 1M people.
That’s not bad considering that doing care home residents must be incredibly slow.
I also wonder how many won’t turn up for the 2nd dose, knowing (?) that the 1st dose is probably really effective on its own.
MedEwok wrote:
AFAIK, the vaccine can be stored at normal fridge temperatures for up to 6 day, so it is all a question of organisation. GP practices in the UK are already vaccinating patients, and they don’t have the ultra-cold fridges either.
Yes, this is certainly the case. Once you remove a batch from -70 storage then you need to have a solid plan in place to make sure you don’t waste it. In extremis there are many ways you can avoid wasting it, such as jabbing all the hospital staff and patients regardless of age/vulnerability, or even (when you are on the last day) taking it to a place where there are a lot of people (a housing estate?) and just running round jabbing it into every arm you can find. The challenge here of course is keeping records of who you jabbed, and the fact that giving someone one jab means a commintment to give them a second jab in ~1 month. As I understand it, they are pulling it out of -70 storage in batches of 700-800 doses, so having a plan about what to do with them in 5-6 days does not represent that big a challenge.
I think it is obvious that supply (rather than logistics or number of jabbers) is the limiting factor. Look at the US, which has already jabbed 2.5x as many people as the UK despite starting at least a week or two later – this must be because they are getting more doses straight from the Pfizer plant(s) in the US whereas the UK depends on delivery from the plant in Belgium.
Yes it is a German product in that BioNTech are the innovators, but Pfizer have picked up all the clinical development costs (they pay the company I work for) and will also be taking care of all the manufacturing – which means they largely call the shots and of course they are a US company. This is how the biotech/pharma partnerships work, because to do something like this you need hundreds of millions of dollars in ready cash – which biotechs do not have. There is a good Panorama programme on the BBC iPlayer about the Oxford vaccine development, and they talk briefly about why they selected AZ to partner with. The unspoken reason, which of course no-one can mention, is that it’s a UK company. Had they partnered with e.g. Roche or Sanofi (and others were sniffing around) then it would have meant a foreign company calling the shots on who gets it first.
It is expected that the MHRA will approve the Oxford/AZ vaccine very soon – today or tomorrow. I would expect this will mean the vaccination rate in the UK will accelerate rapidly, since it is being produced in a plant in North Wales and it doesn’t have the -70 issue. Then we will probably be limited more on logistics and the supply of skilled needle-wielders. It will be interesting to see how much we take for ourselves before we start exporting. Lots of interesting international politics at play here.
As I understand it, the issue that has caused frustration over the slow start in Germany largely comes down to EU solidarity politics. Any EU country could have elected to approve it domestically and start vaccinating (assuming they could get supply) but it appears to have been agreed to wait for EMA approval – “all in this together”.
The militant remainers in the UK (I am a moderate remainer) are all saying that the UK approving it earlier was just posturing and wanting to be seen to be first. Clearly they know nothing about medicines development and regulation (particularly nothing about the international reputation of the MHRA), as they are the same people who talk about ‘cutting corners’ and, hilariously, are the same people who back in the spring said that the UK was condemning its old people to death by opting out of the EU vaccine procurement programme.
Very good post, thank you Graham.
Graham wrote:
Yes it is a German product in that BioNTech are the innovators, but Pfizer have picked up all the clinical development costs (they pay the company I work for) and will also be taking care of all the manufacturing – which means they largely call the shots and of course they are a US company. This is how the biotech/pharma partnerships work, because to do something like this you need hundreds of millions of dollars in ready cash – which biotechs do not have.
Yes, although from what I read, the German government subsidised BioNTech as well, it’s not all Pfizer’s money. Still, you are right, Pfizer is doing the “heavy lifting”, which ultimately benefits the US, as they are a US company. BioNTech has announced they are building another plant in Marburg, Germany, but that won’t be operational for months.
Graham wrote:
As I understand it, the issue that has caused frustration over the slow start in Germany largely comes down to EU solidarity politics. Any EU country could have elected to approve it domestically and start vaccinating (assuming they could get supply) but it appears to have been agreed to wait for EMA approval – “all in this together”.
Once again, this is also correct from my understanding. Germany wanted to avoid “going it alone”, which certainly could have done. Now we have an EU wide approval and a common procurement programme, both of which are good in principle, but apparently there was some dubious stuff going on in the background, with the EU reportedly not buying as much of the vaccine as BioNTech/Pfizer offered them, because the French did not want that “their” domestic vaccine (from Sanofi) is at a disadvantage. Well, Sanofi won’t get an approval for many months, as far as I know.